About R.W. (Rudi) Hendriks, Professor, PhD
Introduction
The Pulmonary Medicine Research Laboratory focuses on fundamental, translational and clinical research concerning the immunopathology of a range of respiratory disorders. In this context, there is a strong interest in the differentiation program of lymphoid cells in health and disease. One of the main aims is to characterize signal transduction pathways and specific transcription factors that induce lymphocyte activation and implement cell fate decisions at specific checkpoints. We explore the involvement of innate lymphoid cells and various T cell subsets in type II immunity, particularly in allergic asthma in human and mouse models. T cell subsets are also studied in a range of other pulmonary disorders, including sarcoidosis, interstitial lung disease and respiratory infection. Furthermore, there is a specific emphasis on signal transduction pathways, in particular B cell receptor signaling, in B cell differentiation, autoimmune inflammation and interstitial lung disease (ILD). In our research, we use state-of-the art technology, including multi-color flow cytometry, multi-omics, imaging mass cytometry, as well as animal models.
Education and career
Rudi W. Hendriks studied Biology at the University of Utrecht, the Netherlands, and performed his PhD studies on X chromosome inactivation patterns in human X-linked immunodeficiency diseases in the Department of Immunohaematology at the Leiden University Medical Center (Prof. J.J. van Rood; Dr. R.K.B. Schuurman) in 1991. Next, he did a post-doctoral training in the Genetics Laboratory of the University of Oxford, UK, studying genetic aspects of X-chromosome inactivation. In 1993 he started his own line of research within the Department of Cell Biology and Genetics of the Erasmus MC (Head: Prof. Frank Grosveld) focusing on the developmental program of B and T lymphocytes in relation to immunodeficiency diseases, cellular activation and leukemia. This research line was continued in the Department of Immunology (Head: Prof. Rob Benner) from 1999 to 2007. After moving to the Department of Pulmonary Medicine in 2007, his research is mainly focused on unraveling the role of lymphocyte populations in chronic airway inflammation.
Recent findings
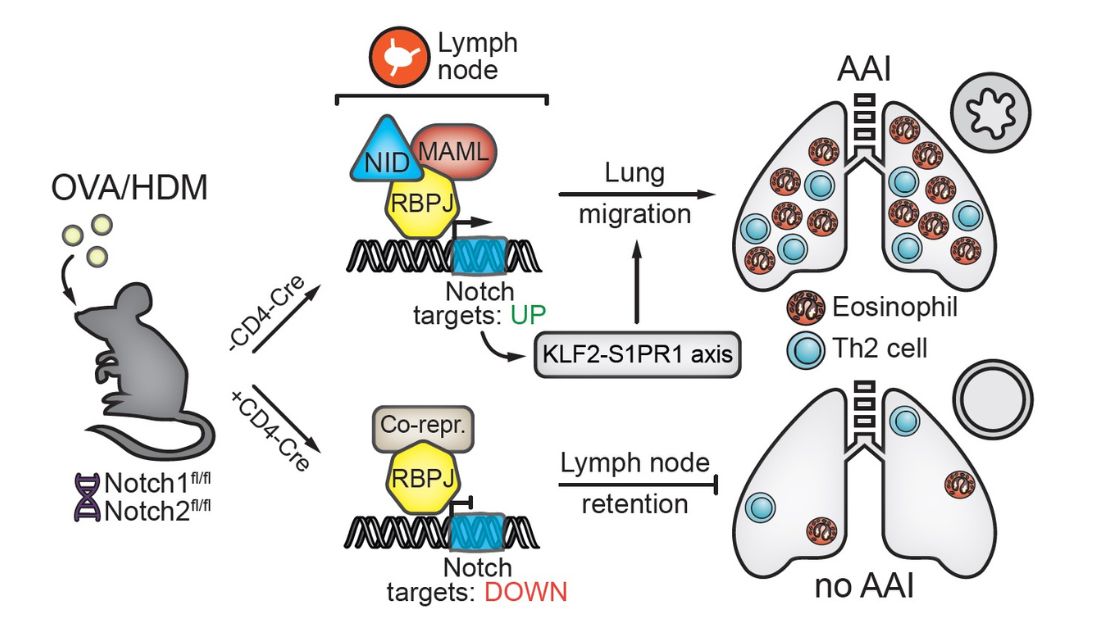
Both T helper 2 (Th2) cells and group 2 innate lymphoid cells (ILC2s) have been implicated in allergic asthma. Using animal models of house-dust mite (HDM)-induced allergic airway inflammation, we found that Notch-deficient Th2 cells do not accumulate in the lung, but are retained in the lung-draining lymph nodes. Transcriptome analysis revealed that Notch signaling licenses allergic airway inflammation by promoting Th2 cell lymph node egress via the KLF2/S1PR1 axis. In HDM-mediated airway inflammation in mice, ILC2s do not provide an early innate source of the pro-inflammatory cytokines IL-5/IL-13, but their activation is dependent on T cells. We observed that T cells and ILC2s are major effectors in influenza-induced exacerbation of HDM-induced allergic airway inflammation in mice. Upon virus clearance, ILC2s regained an activated phenotype and were major contributors to the type 2 cytokine milieu. Recently, we identified type 2 cytokine producing CD8+ T cells as well as an inflammatory CD45RO+ ILC2 subset as major effectors in severe, corticosteroid insensitive, asthma and during asthma exacerbation. Concerning our B cell work, we found that B cell receptor (BCR) signaling is aberrant in a range of systemic autoimmune disorders, pulmonary hypertension as well as pulmonary fibrosis. In particular, protein levels and phosphorylation status of the critical BCR signalling molecule Bruton’s tyrosine kinase (BTK) were increased in B cells. We observed that Btk inhibition – which has impressive efficacy in various B-cell malignancies and autoimmune disorders - induces unexpected.
Selected Publications
- Type-2 CD8+ T cell formation relies on the alarmin interleukin-33 and is linked to asthma exacerbations. Nat Commun (in press).
- Sci Immunol. 6(55):eabd3489
- Rip J, de Bruijn MJW, Neys SFH, Singh SP, Willar J, van Hulst JAC, Hendriks RW, Corneth OBJ (2021). Bruton's tyrosine kinase inhibition induces rewiring of proximal and distal B-cell receptor signaling in mice. Eur J Immunol. 51(9):2251-2265.
- Tindemans I, van Schoonhoven A, KleinJan A, de Bruijn MJ, Lukkes M, van Nimwegen M, van den Branden A, Bergen IM, Corneth OB, van IJcken WF, Stadhouders R, Hendriks RW (2020). Notch signaling licenses allergic airway inflammation by promoting Th2 cell lymph node egress. J Clin Invest. 130(7):3576-3591.
- Li BWS, de Bruijn MJW, Lukkes M, van Nimwegen M, Bergen IM, KleinJan A, GeurtsvanKessel CH, Andeweg A, Rimmelzwaan GF, Hendriks RW (2019). T cells and ILC2s are major effector cells in influenza-induced exacerbation of allergic airway inflammation in mice. Eur J Immunol. 49(1):144-156.
- Corneth OBJ, Verstappen GMP, Paulissen SMJ, de Bruijn MJW, Rip J, Lukkes M, van Hamburg JP, Lubberts E, Bootsma H, Kroese FGM, Hendriks RW (2017). Enhanced Bruton's Tyrosine Kinase Activity in Peripheral Blood B Lymphocytes From Patients With Autoimmune Disease. Arthritis Rheumatol. 69(6):1313-1324.
- Hendriks RW, Yuvaraj S, Kil L (2014). Targeting Bruton’s tyrosine kinase signaling in B cell malignancies. Nature Rev. Cancer 14:219-232.
- Tindemans I, Serafini N, Di Santo JP, Hendriks RW (2014). GATA-3 function in innate and adaptive immunity. Immunity 41:191-206.
- Ribeiro de Almeida C, Stadhouders R, de Bruijn MJW, Bergen IM, Thongjuea S, Lenhard B, Van IJcken W,Grosveld F, Galjart N, Soler E, Hendriks RW(2011). CTCF insulator function limits proximal Vk gene segment recombination and restricts intronic and 3’ enhancer interactions within the immunoglobulin k light chain locus. Immunity 35:501-513.
- Van Loo PF, Dingjan GM, Maas A, Hendriks RW (2007). Surrogate-light-chain silencing is not critical for the limitation of pre-B cell expansion but is for the termination of constitutive signaling. Immunity. 27:468-680.
